Stem Cell Treatment & Research Institution
About nEPS:
Pluripotency of nEPS (newly Elicited Pluripotent Stem Cells without side effects by natural compound)
has been induced from a Mesenchymal Stem Cell.
Free from side effects and immune rejection responses, nEPS is a perfect option for immediate treatment use on anyone. The nEPS discovered using a small molecule named STC-F002, which we had extracted from a natural compound.
Good for
Immediate
Human Use
No Side Effects
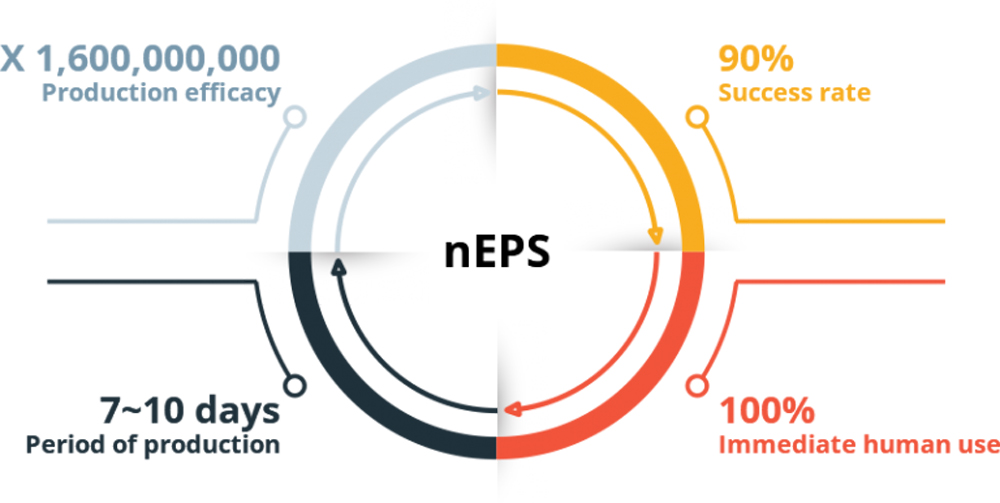
Up to
X1,600,000,000
Production
Efficacy
Inducing Pluripotency:
The nEPS is a pluripotent stem cell without side effects induced from separating human mesenchymal stem cells
(hMSCs) of the umbilical cord, umbilical cord blood, bone marrow, or adipose tissue.
The hMSCs are cultured and then treated with a small molecule compound (F002) extracted from a natural product. In such a case, hMSCs can form many colonies that show the expression of DNA genes (Figure 1) only found in
pluripotent stem cells.
The adult stem cell can only be differentiated into cells of a specific tissue but pluripotent stem cell has a core advantage as a cell therapy product as they can be differentiated intoall cell sorts human body tissues including ectodermal, endodermal and mesodermal cells.
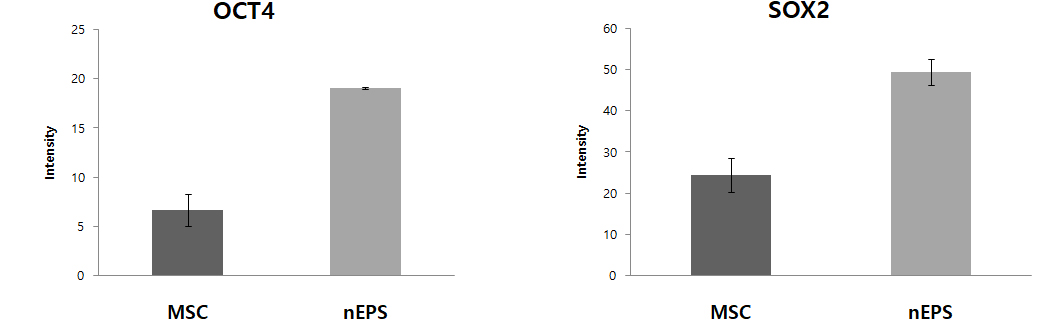
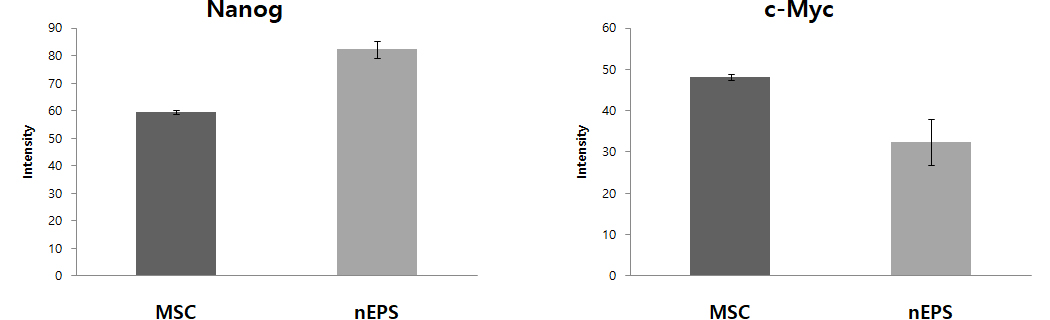
Fig. 1 nEPS-treated groups show the expression of protein and DNA genes. OCT4, SOX2, Nanog, and c-Myc are major genes of pluripotent stem cells. Expression of OCT4, SOX2, Nanog, the core genes of pluripotent stem cells, were remarkably increased in the nEPS group, while the gene related to the formation of the tumor, c-Myc was reduced.
What we have achieved so far:
– nEPS group has lower c-Myc gene expression, which is related to tumor occurrence, compared to the MSC group.
– STRI hadconducted a carcinogenicity study to investigate the effect of nEPS compared to MSC on seminal vesicle of SCID(Severe combined Immune Deficiency) mouseat Yonsei University.Biopsy tests performed did not show any sign of tumor occurrence even until 20 weeks post nEPS treatment.
Safety & Mass Production Enabled:
The nEPS can be used on anyone without immunological rejection. Which means it can be used as allogeneic and autologous MSCs.
It shows significantly reduced time for production (production efficiency of over 90%), and more importantly, perhaps it is free from side effects, including tumors which are caused by mutation of the gene sequence from gene manipulation, since nEPS pluripotent gene expression is increased by DNA methylation using natural products.